
Specimens in anhydrous glycerin as of 8/7/17. They were transferred to glycerin on 6/31.
Sodium borate solution was changed and fresh trypsin digest was replaced

On 7/17, the toluidine blue and acid fuchsin specimens had lightened once more of the stain had leached out

On 7/17, excess stain had leached out

Results from 7/10. Specimens were transferred from their respective stains to fresh trypsin digest

Results from 7/10. Alizarin red selectively stained the bones and scales

Toluidine blue


Results from 7/10. Because of the Fourth of July, I hadn't visited the lab in two weeks so the stain permeated the fish

Results from 7/10


Of the 7 fish that had not been stained with alizarin, 4 were transferred to toluidine blue and 3 to acid fuchsin

These are new experimentations to bypass the lack of alcian blue. Toluidine blue solution (left) consists of 0.22 g stain, 30% acetic acid, and 70% EtOH. Acid fuchsin solution (right) contains 1 g stain per every 1 ml glacial acetic acid and 100 ml distilled water.

By 6/26, the fish had been stained, including the scales (which happened in 2014 when I omitted the descaling step). Interestingly, the portion of the tail that stuck out of the solution was NOT stained, giving me a new idea which I will explore later. One jar of specimens was transferred to trypsin while the rest were kept in the stain to test the difference in staining intensity.

On 6/19, I transferred 10 of the specimens to alizarin red (since the lab has no alcian blue). The remaining 7 were left in trypsin.

Fish are considerably lighter and have been returned to fresh trypsin digest.

Specimens were transferred to bleaching solution which, according to one protocol, calls for 15% hydrogen peroxide and 85% potassium hydroxide. Improvising again, I prepared 300 ml of 1% KOH and added around 100 ml of 30% hydrogen peroxide (instead of traditional 3%). Specimens sat in this solution for 40 minutes with a halfway check.

On 6/12, specimens had sunk with insignificant clearing. At this point, I decided to bleach them considering that the silver band might obscure the skeletal structure.
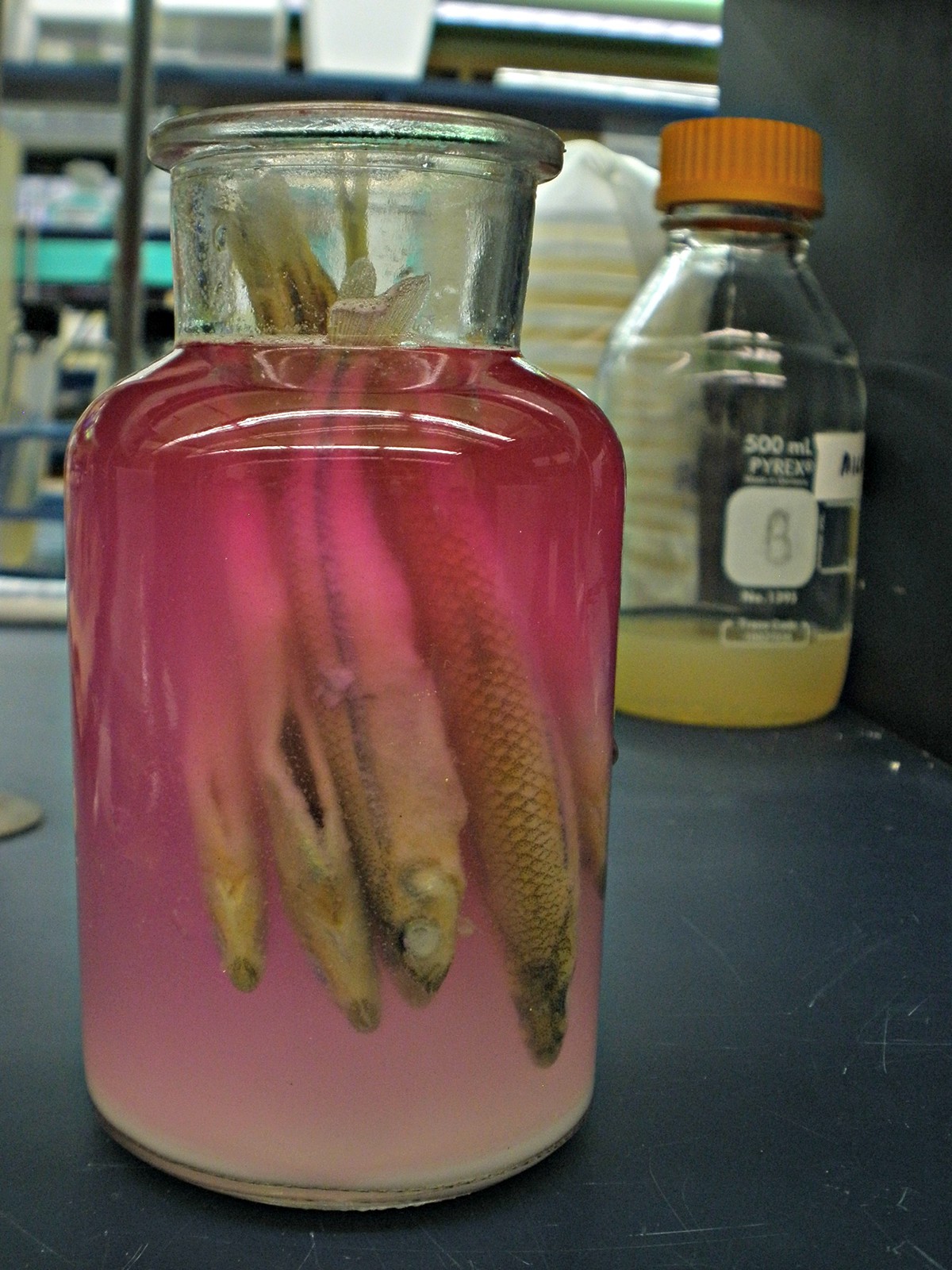
Fish were transferred to trypsin digest on 6/5. Initially, I skipped the bleaching step after attempting to descale the specimens. This species does not seem to have flaky scales. I also omitted the alcian blue staining since the lab did not stock it. Trypsin EDTA solution for cell culture was substituted for porcine trypsin powder. The solution was balanced with phenol red, hence the fish pink sangria.

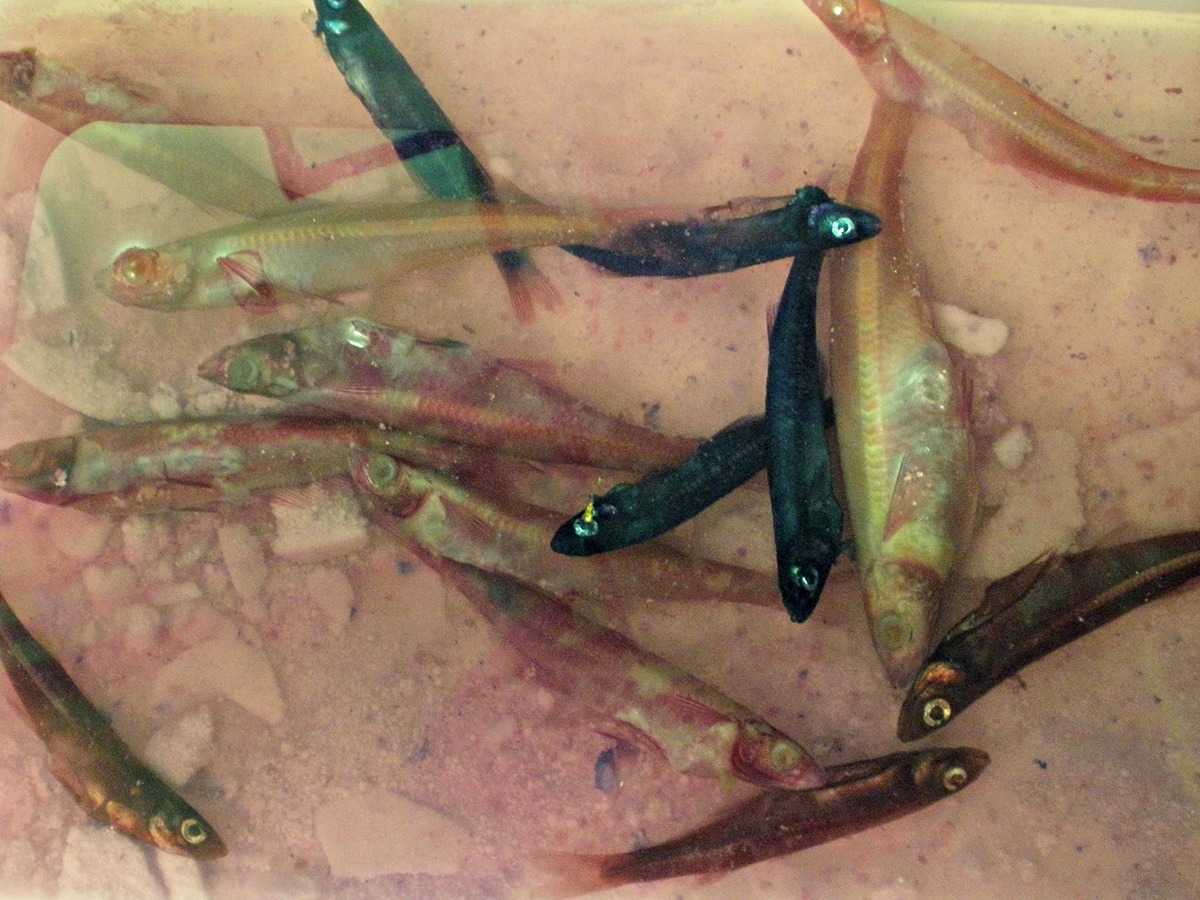
Specimens after evisceration, formalin fixation, and dehydration. Many improvisations were made due to time constraints (I only visit the lab once a week). Specimens were eviscerated and fixed on 5/15, transferred to distilled water on 5/22, and transferred to 90% EtOH on 5/29.


Atlantic silverside specimens left over from another student's thesis project. In total, I selected 17 specimens for this round.

